
The U.S. Food and Drug Administration has initiated a fundamental transformation of its regulatory oversight for digital health, ushering in an era where many low-risk technologies no longer require stringent premarket review. This strategic deregulation, detailed in guidance issued this year, is
The United Kingdom's healthcare landscape has long presented a paradox for medical technology innovators, where groundbreaking devices and diagnostics capable of transforming patient outcomes often face a fragmented and unpredictable journey to widespread adoption within the National Health Service
As an expert in robotics and IoT applications in medicine, James Maitland has dedicated his career to a powerful, singular passion: leveraging technology to advance healthcare solutions. Yet, he cautions that the industry's rush toward futuristic concepts like AI and virtual hospitals often

After years of meteoric growth that redefined the landscape for innovative medical technology, the U.S. Food and Drug Administration's (FDA) Breakthrough Devices Program has settled into a new, more predictable rhythm. This shift from rapid acceleration to a consistent, high-volume cadence signals

A diagnostic imaging machine, having faithfully served a decade in a European hospital, now presents a complex quandary for Indiis it a cost-effective tool for expanding healthcare to underserved communities or a potential danger to patient safety that undermines domestic industry? This dilemma
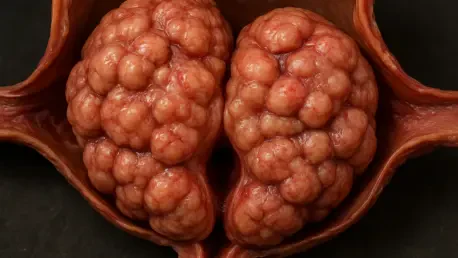
For millions of aging men, the diagnosis of Benign Prostatic Hyperplasia, or an enlarged prostate, presents an unwelcome crossroads between enduring disruptive urinary symptoms and accepting treatments with significant drawbacks. This condition affects an estimated 50% of men between 51 and 60, a