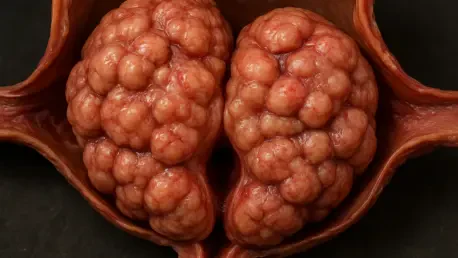
For millions of aging men, the diagnosis of Benign Prostatic Hyperplasia, or an enlarged prostate, presents an unwelcome crossroads between enduring disruptive urinary symptoms and accepting treatments with significant drawbacks. This condition affects an estimated 50% of men between 51 and 60, a

The silent closure of a rural hospital sends ripples far beyond its empty corridors, leaving communities without critical access to care and threatening the local economy. With an unnerving 146 rural hospitals shutting their doors since 2005 and another 700 teetering on the brink of closure, the

A federal jury has delivered a verdict that sends shockwaves not just through the medical device industry but across the landscape of corporate competition, handing down a penalty worth over a third of a billion dollars not for a faulty product but for the way it was sold. This case highlights how

The monumental challenge of delivering consistent, empathetic support across millions of annual customer interactions has long been a significant hurdle for the healthcare industry. Humana's Agent Assist, a sophisticated AI tool developed with Google Cloud, enters this landscape not as a

A New Era of Trade: A Breakthrough for Global MedTech A seismic shift in international trade policy has just unlocked unprecedented opportunities for India's medical technology sector, following a landmark agreement with the United States to dramatically reduce import tariffs. Hailed by industry

The seamless access to patient data that modern healthcare professionals now take for granted was once a distant dream, a concept forged in an era of cumbersome paper files and handwritten charts. The recent passing of A. Neil Pappalardo on January 27 at the age of 83 marks the end of an era for