
After years of meteoric growth that redefined the landscape for innovative medical technology, the U.S. Food and Drug Administration's (FDA) Breakthrough Devices Program has settled into a new, more predictable rhythm. This shift from rapid acceleration to a consistent, high-volume cadence signals
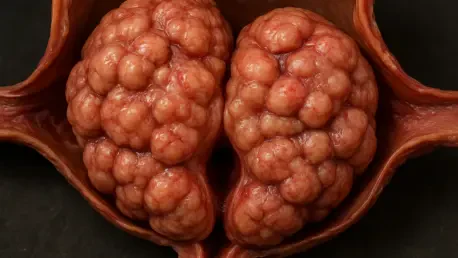
For millions of aging men, the diagnosis of Benign Prostatic Hyperplasia, or an enlarged prostate, presents an unwelcome crossroads between enduring disruptive urinary symptoms and accepting treatments with significant drawbacks. This condition affects an estimated 50% of men between 51 and 60, a

The silent closure of a rural hospital sends ripples far beyond its empty corridors, leaving communities without critical access to care and threatening the local economy. With an unnerving 146 rural hospitals shutting their doors since 2005 and another 700 teetering on the brink of closure, the
In the global pursuit of more resilient and accessible healthcare systems, nations are increasingly looking beyond their borders for proven models of technological integration. A significant delegation from South Korea’s National Health Insurance Service (NHIS) recently traveled to Washington D.C.,

A significant career change often brings excitement and opportunity, but for millions of Americans, it also triggers a silent crisis: the loss of their trusted therapist. In a healthcare system where mental health benefits are frequently tied to employment, life transitions like a new job or a

The silent pandemic of chronic diseases, responsible for a staggering 73% of all deaths worldwide, is placing an unprecedented strain on modern healthcare systems. In nations like India, where non-communicable diseases (NCDs) contribute to over 53% of all fatalities and 44% of disability-adjusted
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31