In a striking revelation that has sent ripples through the beauty and healthcare sectors, a recent investigative report by a major national broadcaster in China has uncovered disturbing practices within the cosmetic injection industry, particularly surrounding popular treatments like water-light injections designed to enhance skin hydration and appearance. This exposé has brought to light deceptive tactics employed by some hospital staff and clinics, raising urgent questions about consumer safety and the adequacy of regulatory frameworks. With the medical aesthetics market booming as more individuals seek non-surgical enhancements, the potential risks tied to unethical behavior and inadequate oversight have become a pressing concern. The findings not only challenge the trust placed in these procedures but also highlight a critical need for reform to protect vulnerable consumers from hidden dangers lurking beneath the promise of beauty.
Uncovering the Hidden Risks
Deceptive Practices in Device Classification
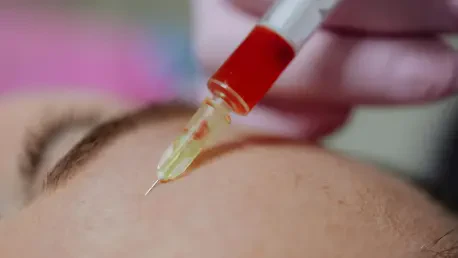
The core of the issue lies in the deliberate misrepresentation of medical device classifications by certain industry players, a practice that undermines the safety of cosmetic injections. Hospital staff, including high-ranking directors and consultants, have been found to mislead consumers by asserting that any device bearing a medical device registration number is suitable for invasive procedures. This obfuscation ignores the crucial distinction between Class II and Class III devices, with the latter requiring stringent regulatory approval due to their higher risk and invasive nature. Instead, some clinics have resorted to using Class II devices—intended for less invasive applications—for treatments like water-light injections, exposing patients to significant health hazards. Such actions reveal a troubling gap in ethical standards and suggest that profit motives often take precedence over consumer well-being in certain segments of the industry, necessitating immediate attention to prevent further harm.
Health Implications for Unsuspecting Consumers
Beyond the ethical breaches, the health implications of using misclassified devices in cosmetic procedures are deeply concerning and cannot be overlooked. When treatments are administered with improper equipment, the risk of complications such as infections, severe allergic reactions, and even permanent disfigurement rises dramatically. Public discourse on social media platforms has amplified these fears, with many sharing personal stories of adverse effects and drawing parallels to high-profile cases of celebrity health issues linked to aesthetic treatments. This growing unease reflects a broader recognition of the vulnerabilities faced by individuals seeking beauty enhancements, often without full awareness of the potential dangers. The lack of transparency about the tools used in these procedures further compounds the problem, leaving consumers exposed to risks they neither anticipate nor understand, and highlighting an urgent need for better safeguards.
Addressing Systemic Challenges
Regulatory Loopholes and Enforcement Gaps
A deeper examination of the cosmetic injection industry reveals systemic challenges rooted in regulatory loopholes and insufficient enforcement mechanisms that allow unethical practices to persist. The existence of a “gray area” in medical aesthetics—where unapproved or misclassified products are routinely used—points to unclear guidelines and inconsistent oversight. Even institutions perceived as reputable have been implicated in misleading consumers, suggesting that the problem is not confined to rogue operators but is indicative of broader industry flaws. This situation is exacerbated by the absence of robust penalties for non-compliance, which fails to deter clinics from prioritizing financial gain over safety. As public health concerns mount with the potential for serious adverse reactions, there is a clear imperative for authorities to tighten market surveillance and establish stricter licensing requirements to ensure that only qualified practitioners and approved devices are utilized.
Building Consumer Trust Through Awareness
Equally critical to addressing these systemic issues is the need to empower consumers with knowledge to make informed decisions about cosmetic treatments. Many individuals remain unaware of the risks associated with procedures like water-light injections or the importance of verifying practitioner credentials and product legitimacy. The public outcry following the recent exposé, particularly on platforms like Weibo, underscores a widespread sentiment of distrust, with some dismissing these treatments as deceptive or ineffective. Enhancing consumer education about the distinctions between medical device classifications and the potential hazards of unverified treatments could serve as a powerful tool in rebuilding confidence. Additionally, fostering transparency regarding product sourcing and the qualifications of practitioners is essential to counteract the misinformation that currently pervades the industry, ensuring that those seeking aesthetic enhancements are not left vulnerable to exploitation.
Moving Toward Safer Practices
Reflecting on Past Oversights
Looking back, the investigative report that exposed the misuse of medical devices in China’s cosmetic injection industry marked a pivotal moment in sparking national dialogue about safety and ethics. It revealed a troubling pattern of oversight failures, where ethical misconduct by some practitioners went unchecked, leaving countless consumers at risk due to misinformation and inadequate regulation. The public’s vocal response on social media platforms highlighted a collective frustration with an industry that often prioritized profit over patient well-being. These past shortcomings served as a stark reminder of the vulnerabilities inherent in a rapidly growing sector lacking sufficient accountability, prompting a reevaluation of how such treatments were administered and regulated.
Charting a Path for Reform
Moving forward, the focus must shift to actionable reforms that address the root causes of these safety concerns and prevent future lapses. Strengthening regulatory frameworks to eliminate gray areas in device classification and procedure guidelines should be a priority for authorities. Implementing harsher penalties for non-compliant clinics, alongside enhanced training programs for practitioners, could deter unethical behavior and elevate industry standards. Furthermore, public education initiatives must be expanded to raise awareness about the risks of cosmetic injections and the importance of choosing verified providers. As the government intensifies its efforts to curb deceptive practices, these steps could pave the way for a safer, more transparent medical aesthetics sector, restoring consumer confidence and protecting public health from the hidden dangers that once prevailed.