In the silent, swift moments after a major stroke, when a blood clot lodges in a major artery of the brain, the path to recovery is measured not in days or hours, but in precious, irreplaceable minutes. For countless individuals in India, the most effective medical intervention has long remained an inaccessible luxury, a reality that is now poised for a monumental shift following the successful clinical trial and regulatory approval of a domestically engineered life-saving device. This breakthrough represents more than just a medical advancement; it signals a new era of self-reliance and equitable healthcare for one of the world’s most populous nations.
When a Stroke Strikes What if the Best Treatment Was Also the Most Affordable
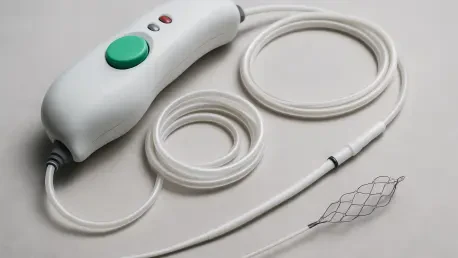
The gold standard for treating severe ischemic strokes, known as large vessel occlusions (LVO), is a sophisticated procedure called mechanical thrombectomy. During this intervention, physicians navigate a catheter through the body’s arteries to the brain, deploying a tiny, cage-like device known as a stent retriever. This instrument is designed to ensnare the blood clot and physically remove it, rapidly restoring vital blood flow. The success of this procedure is profoundly time-sensitive; every second that an artery remains blocked, millions of brain cells die, increasing the risk of permanent disability or death. The procedure itself is a marvel of modern medicine, offering the best chance for a patient to regain functional independence.
However, the availability of this life-saving technology has been severely constrained by economic realities. The stent retrievers used in India have historically been imported, carrying a price tag that places them far beyond the reach of the average citizen. This created a stark disparity in healthcare, where the ability to receive the most effective treatment was often determined by financial status rather than medical need. The dream of making this advanced care a standard, accessible option for all who need it has fueled a decade of research and development within India, culminating in a solution designed to break down this cost barrier once and for all.
The Critical Treatment Gap India’s High Stakes Battle Against Stroke
The challenge posed by stroke in India is staggering in its scale. Each year, an estimated 1.7 million people in the country suffer a stroke, making it a leading cause of death and long-term disability. This public health crisis places an immense burden not only on patients and their families but also on the entire healthcare system. For those who survive a severe stroke, the road to recovery can be long and arduous, often resulting in a permanent loss of independence and a significant decline in quality of life. The economic impact is equally devastating, encompassing high medical costs, lost productivity, and the need for long-term care, creating a cycle of hardship for many households.
This national health crisis was exacerbated by the prohibitive cost of the very technology designed to combat it most effectively. The reliance on imported stent retrievers created a critical treatment gap, where thousands of eligible patients were denied access to mechanical thrombectomy simply because of its price. Hospitals, particularly those outside major metropolitan centers, struggled to stock these expensive devices, further limiting their availability. This disparity effectively rendered the best-in-class stroke treatment an exclusive option, leaving a vast majority of the population to face the consequences of a severe stroke without access to the intervention most likely to save their brain and their future.
The GRASSROOT Trial Putting a Homegrown Solution to the Ultimate Test
To bridge this gap, Indian engineers and clinicians developed the Supernova stent retriever, a “Made in India” device designed to deliver world-class performance at a fraction of the cost. To prove its mettle, the device was subjected to a rigorous, multicenter clinical trial named GRASSROOT (Gravity Stent-Retriever System for Reperfusion of Large Vessel Occlusion Stroke Trial). Coordinated by the prestigious All India Institute of Medical Sciences (AIIMS) in New Delhi, the study was conducted across eight specialized stroke centers, ensuring a diverse and representative patient population. The trial enrolled 32 patients suffering from severe LVO strokes, with an average age of 58 and a significant presence of common risk factors like hypertension and diabetes, mirroring real-world clinical scenarios.
The results of the GRASSROOT trial were nothing short of a resounding success, with outcomes that stand shoulder-to-shoulder with established international standards. The primary goal of the procedure—restoring blood flow to the brain, or reperfusion—was successfully achieved in nearly 94% of patients. This remarkable success rate was often accomplished with impressive efficiency, frequently requiring only one or two attempts to clear the blockage. This efficiency is critical, as faster reperfusion directly correlates with better patient outcomes. Furthermore, the device demonstrated an excellent safety profile, with low incidences of mortality and serious complications, and, most importantly, no adverse events were reported to be directly related to the Supernova device itself.
The long-term benefits for patients were equally compelling. At the 90-day follow-up mark, a standard milestone for assessing stroke recovery, half of the trial participants had achieved functional independence, meaning they could manage their daily activities without assistance. This statistic represents a profound victory, translating to lives restored and families kept whole. The trial’s robust methodology and stellar outcomes earned it publication in the highly respected Journal of Neurointerventional Surgery, a BMJ group journal, providing international validation of its findings and cementing its credibility within the global medical community.
A Turning Point for India Expert Voices on a Historic Medical Achievement
Leading medical experts have unanimously hailed the GRASSROOT trial as a landmark achievement. Dr. Shailesh B. Gaikwad, the national principal investigator from AIIMS, highlighted its significance in proving India’s capability to conduct and generate its own world-class clinical evidence for advanced medical technologies. He emphasized that this success reduces the nation’s long-standing reliance on foreign-led research and expensive imported devices, marking a pivotal moment in India’s journey toward medical self-sufficiency. This trial has established a robust framework that can be replicated for future domestic innovations.
The global implications of this development were underscored by Dr. Dileep Yavagal, the trial’s global principal investigator. He noted that an affordable, domestically manufactured stent retriever has the potential to democratize access to this critical therapy not only within India but also in other low- and middle-income countries facing similar challenges. This sentiment was shared by Dr. Deepti Vibha of AIIMS, who credited the invaluable contribution of the patients and their families, whose participation was essential in bringing faster and more affordable stroke therapies closer to reality for millions.
Looking forward, Dr. Ashutosh Jadhav of Gravity Medical Technology, the company behind the device, stated that the trial has laid a formidable foundation for the future of India-led clinical research. This success is seen not as a final destination but as a crucial stepping stone. It provides the confidence and the blueprint for undertaking more ambitious, large-scale clinical trials for other homegrown medical devices, fostering a vibrant ecosystem of innovation within the country and signaling a new era of leadership in the global med-tech landscape.
From Clinical Trial to National Rollout The Far Reaching Impact of the Supernova Stent
Armed with compelling, domestically generated data from the GRASSROOT trial, the Central Drugs Standard Control Organisation (CDSCO) granted full approval for the routine clinical use of the Supernova stent retriever. This decision is historic, as it marks the first time a stroke device has been cleared in India based entirely on a national trial. This sets a powerful new precedent for other Indian medical innovators, demonstrating a clear and viable pathway from concept to clinical reality without depending on foreign validation, a significant victory for the “Make-in-India” initiative.
The far-reaching impact of this approval is poised to reshape the landscape of stroke care across the nation. By boosting self-reliance in a critical area of advanced healthcare, India has taken a major step toward insulating its healthcare system from the vulnerabilities of global supply chains and price fluctuations. The domestic manufacturing of the Supernova stent will not only ensure a stable supply but will also cultivate a skilled workforce and stimulate further investment in the high-tech medical device sector, creating a positive feedback loop of growth and innovation.
The path forward is now focused on ensuring this groundbreaking technology reaches every corner of the country. The successful trial and subsequent approval have paved the way for the national rollout of an affordable, effective treatment that could benefit a significant portion of the 1.7 million people who suffer strokes each year. The GRASSROOT trial did more than just validate a medical device; it ignited a beacon of hope and inspired a new wave of Indian med-tech pioneers. It demonstrated that with vision, collaboration, and rigorous science, India could not only solve its own healthcare challenges but also contribute powerful, accessible solutions to the world.