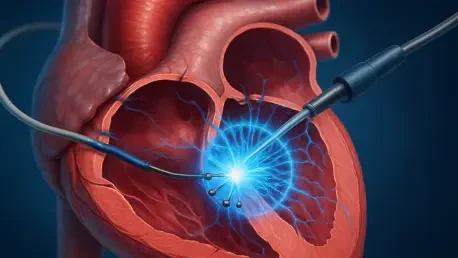
Imagine a world where life-threatening conditions like atrial fibrillation or cancer can be treated with pinpoint precision, minimal risk, and faster recovery times, thanks to the rapid evolution of pulsed field ablation (PFA) and pulsed electric field treatments. These technologies are reshaping

Imagine a world where managing chronic illnesses no longer means frequent hospital visits or cumbersome injections, but instead involves a discreet, wearable device that delivers life-sustaining medication seamlessly over hours or even days. Wearable injectors, innovative medical tools designed as

What happens when the very people tasked with saving lives are silently breaking under the weight of their own burdens? In hospitals across the globe, healthcare workers face relentless pressure, often with little support to manage the emotional toll. A staggering statistic from recent studies

What if the key to halting a global health crisis costing millions of lives annually rests in a device smaller than a credit card and cheaper than a latte? Antimicrobial resistance (AMR), a silent yet deadly threat, claims countless victims by rendering antibiotics ineffective against bacterial
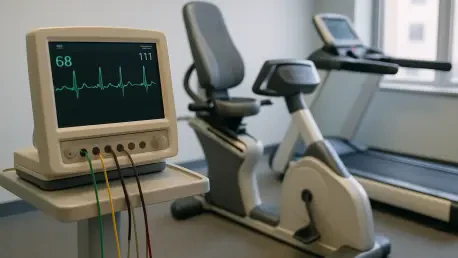
In an era where heart disease continues to be a leading cause of mortality across the globe, the importance of effective recovery solutions has never been more evident, and the Cardiac Rehabilitation Device Market stands as a critical pillar in healthcare. This market offers innovative tools
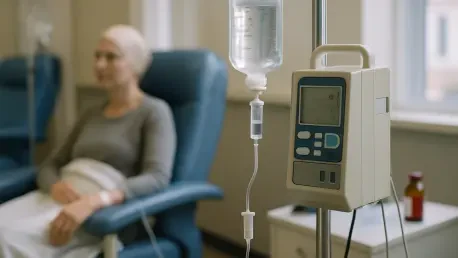
In the heart of Sanford, Florida, a remarkable story of innovation and impact unfolds through the efforts of a high-tech manufacturing company dedicated to transforming cancer treatment. .Decimal, a firm specializing in medical devices, has emerged as a beacon of hope for patients battling this