
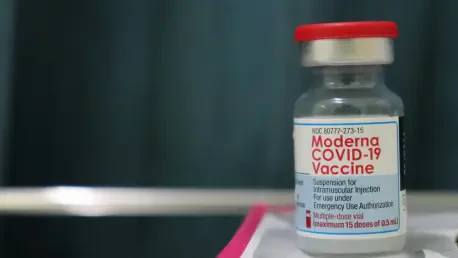
The global pharmaceutical landscape has undergone a seismic shift following the formal announcement that Moderna has agreed to a massive two-point-two-five billion dollar settlement with Genevant Sciences and Arbutus Biopharmaceutical to resolve long-standing disputes over lipid nanoparticle

The recent surge in kinetic military operations throughout the Tehran metropolitan area has revealed a devastating level of vulnerability within the civilian medical infrastructure that serves millions of residents. As military strikes targeted strategic installations in early 2026, the resulting

The rapid push toward federal health information technology deregulation promises to streamline administrative workflows, but it simultaneously threatens to leave the most vulnerable segments of the medical landscape behind in a wave of technical obsolescence. As the Assistant Secretary for

The intersection of chronic disease management and health insurance reform is reaching a critical tipping point in Ohio as hundreds of thousands of residents grapple with the complexities of long-term illness. While the traditional medical model often prioritizes acute interventions during moments

The Colorado Joint Budget Committee currently faces the daunting task of reconciling a massive one billion dollar state budget shortfall with the urgent and increasing demand for accessible mental health services across the region. As federal Medicaid contributions dwindle, state lawmakers find
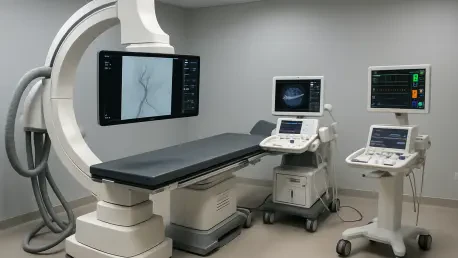
The delivery of sophisticated medical technology to the High-Specialty Regional Hospital of the Yucatán Peninsula in Mérida represents a transformative shift in the region's ability to provide life-saving care to its most vulnerable populations. Governor Joaquín Díaz Mena spearheaded this

The American healthcare landscape is currently grappling with a systemic crisis characterized by a sharp rise in insurance claim denials and administrative barriers that prevent timely medical intervention. While health insurance is fundamentally intended to provide a financial and medical safety

The successful reintegration of formerly incarcerated individuals into their communities remains one of the most significant challenges facing the modern American criminal justice system today. Statistics consistently indicate that the first few months following release are the most volatile, as

A Tale of Two Tickers: When Record Profits Meet Investor Skepticism The morning of February 18, 2026, began with a striking divergence between the cold, hard numbers on a corporate balance sheet and the volatile reactions of the public trading floor. What happens when a global healthcare giant

The global medical scrubs industry is currently undergoing a massive transformation as healthcare facilities worldwide prioritize both staff comfort and strict infection control protocols. With the market projected to reach a valuation of $85.8 billion by 2033, the trajectory reflects a steady
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy