

Imagine a typical weekend where the allure of late-night socializing or binge-watching a favorite series keeps bedtime at bay, only to be followed by the tempting prospect of sleeping in the next morning. For many, this shift in routine feels like a well-deserved break from the rigors of the

The passage of the One Big Beautiful Bill Act (OBBB Act) on July 4 of this year has unleashed a seismic shift in the U.S. healthcare landscape, promising federal savings of $326 billion over the next decade while introducing severe Medicaid work requirements that could reshape the system. This

Imagine being an undergraduate student stepping into a world-class research facility, surrounded by cutting-edge technology and brilliant minds, with the chance to contribute to groundbreaking medical studies. This is the reality for many students who participate in Research Experiences for
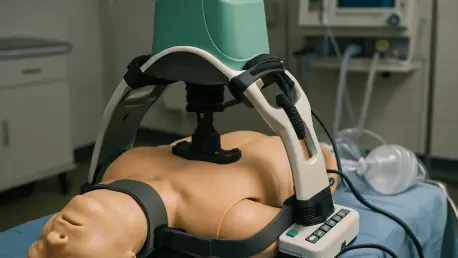
In rural America, where vast distances and sparse populations define the landscape, the challenge of responding to out-of-hospital cardiac arrest (OHCA) is a daunting reality for emergency medical services (EMS). These communities often grapple with delayed response times, limited staffing, and

In a transformative stride for public health, Aotearoa New Zealand has recently elevated its National Healthy Food and Drink Policy from a voluntary guideline to a mandatory standard in public hospitals as of June this year. This pivotal change targets the food environment for hospital staff and
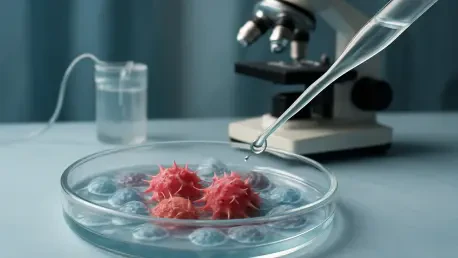
In a significant stride toward better outcomes for patients grappling with one of the most aggressive forms of breast cancer, a groundbreaking clinical study from South Korea has emerged as a beacon of hope for those affected. Known as the MIRINAE trial, or KCSG-BR18-21, this phase II randomized

In the small town of Shutesbury, Massachusetts, a financial storm is brewing as health insurance costs have surged dramatically, placing an unprecedented burden on both municipal budgets and local employees. With premiums skyrocketing by nearly 40% in a short span due to consecutive increases
I'm thrilled to sit down with James Maitland, a renowned expert in robotics and IoT applications in medicine, whose passion for integrating technology into healthcare has led to groundbreaking insights. With a deep understanding of how policy and innovation intersect, James is the perfect person to

In a region where tropical climates foster the spread of vector-borne diseases, Cuba stands at the forefront of a persistent public health challenge, grappling with multiple viral outbreaks that threaten the well-being of its population. Recent updates from Francisco Durán García, the national

Imagine a senior citizen, after decades of hard work, facing the daunting reality of choosing between paying for life-saving medications and covering basic living expenses like rent or groceries, a scenario that is not a distant fear but a daily struggle for millions of low-income Medicare
ITCurated uses cookies to personalize your experience on our website. By continuing to use this site, you agree to our Cookie Policy