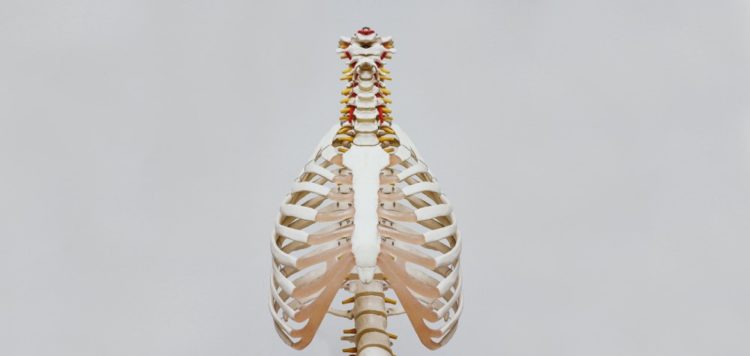
The US Food and Drug Administration (FDA) has granted an Investigational Device Exemption (IDE) to AgNovos Healthcare’s latest investigational product, the AGN1 Local Osteo-enhancement Procedure (LOEP) Small Volume (SV) kit.
This kit is an investigational device for treating stable but painful vertebral compression fractures through a less-invasive procedure. It was formally granted a breakthrough device designation from the FDA.
The IDE for the AGN1 LOEP SV kit will allow a study to be started to analyse the device’s ability to lower pain and support mobility in vertebral compression fracture patients.