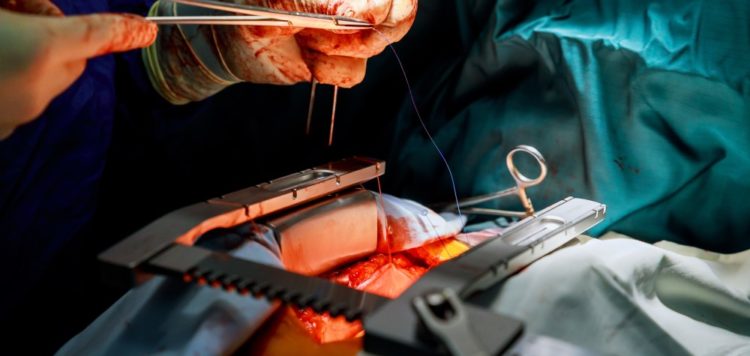
Thubrikar Aortic Valve has concluded the preliminary CE Mark-enabling study of the Optimum Transcatheter Aortic Valve (Optimum TAV) in severe aortic stenosis patients.
The Competent Authority of Poland granted approval for the five-subject study, which was carried out in line with the European Union Medical Device Regulation (EU MDR) and observed by KCRI, a clinical research organisation (CRO).
The company noted that better single-digit mean gradients and effective orifice areas (EOAs) were observed compared to the majority of transcatheter aortic valve replacement (TAVR) valves currently available in the market.